
These cells have flat, monolayered colony morphology ( Figure 2), rely on basic fibroblast growth factor ( bFGF)/Activin A signaling for self-renewal, are refractory to single-cell colonization, and have gone through X chromosome inactivation in females (XaXi) ( Thomson et al., 1998 Vallier et al., 2004 James et al., 2005 Camus et al., 2006). The postimplantation pluripotent state is represented by primed pluripotency ( Brons et al., 2007 Tesar et al., 2007 Nichols and Smith, 2009 Najm et al., 2011) as seen in human ESCs/iPSCs and mouse epiblast stem cells. Pluripotency is classified into at least three different stages. Upon implantation, ICM from the blastocyst will differentiate into primitive endoderm and epiblasts, with the latter further developing into the three-germ layers of an embryo, while the trophoblasts will become the placenta. Blastomeres isolated from the ICM of mammalian embryos could be cultured in vitro to generate pluripotent ESCs. After that, it will form a blastocyst, consisting of trophoblasts, blastocyst cavity, and the ICM. Then, it enters the cleavage process and divides into two cells, four cells, eight cells, etc., and to morula stage. The embryo development starts from a zygote. The development of early mammalian embryos. Since the initial introduction by Yamanaka’s group, the iPSCs technology has been successfully applied to many species from rodents to primates ( Liu et al., 2008 Esteban et al., 2009 Li et al., 2009, 2011b Liao et al., 2009 Wu et al., 2009 Honda et al., 2010 Tomioka et al., 2010 Bao et al., 2011 Nagy et al., 2011 Breton et al., 2013).

Compared with ESCs, iPSCs have multiple advantages in terms of generation and applications, including the unnecessity of expensive instrumentation and labor-intensive process, the ability to be mass produced in the laboratory environment within short timeframes, the convenience of autologous iPSCs derivation from host somatic cells (host-specific iPSCs), and the circumvention of the ethical dilemmas underlying destruction of human and animal embryos. These cells were initially reprogrammed from mouse and human somatic cells by the exogenous expression of OCT4 (also known as POU5F1), SOX2, KLF4, and c-MYC ( OSKM), or OCT4, SOX2, NANOG, and LIN28 ( OSNL) ( Takahashi and Yamanaka, 2006 Okita et al., 2007 Yu et al., 2007).
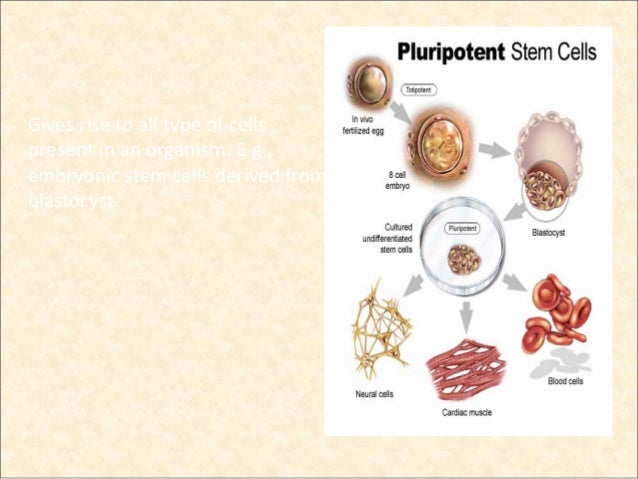
Since 2006, another type of PSCs, the induced pluripotent stem cells ( iPSCs) rapidly emerged. These properties of PSCs are fundamental for the application in regenerative medicine, animal reproduction, and modeling diseases. Pluripotent stem cells ( PSCs) were mainly referring to embryonic stem cells ( ESCs) derived from the inner cell mass ( ICM) of animal embryos ( Figure 1), which can differentiate into any cell type of the three-germ layers and can propagate almost infinitely in a laboratory setting ( Martin, 1981 Bradley et al., 1984 Thomson et al., 1998 Gilbert, 2013). We also discuss the problems associated with the farm animal iPSCs and potential future directions toward the complete reprogramming of somatic cells from farm animals.
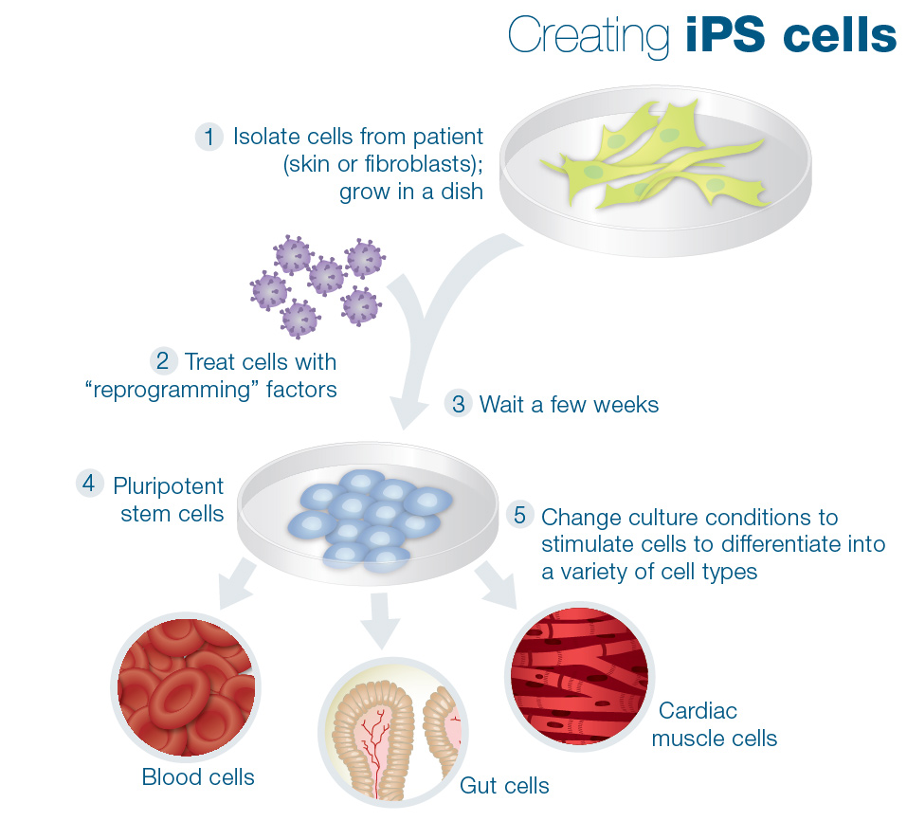
In this review, we summarize the history and current progress in domestic farm animal iPSC generation, with a focus on swine, ruminants (cattle, ovine, and caprine), horses, and avian species (quails and chickens). The most prominent of them remain the inability of these cells to silence exogenous reprogramming factors, the obvious reliance on exogenous factors for their self-renewal, and the restricted development potential in vivo. Although much progress has been made toward the generation of PSCs from these species, major obstacles remain precluding the exclamation of the establishment of bona fide iPSCs. Induced PSCs from domesticated animals thus harbor enormous scientific, economical, and societal values. Generation of iPSCs from domesticated animals would provide unrestricted cell resources for the study of embryonic development and cell differentiation of these species, for screening and establishing desired traits for sustainable agricultural production, and as veterinary and preclinical therapeutic tools for animal and human diseases. The development of the induced pluripotent stem cells ( iPSCs) technology has revolutionized the world on the establishment of pluripotent stem cells ( PSCs) across a great variety of animal species.


 0 kommentar(er)
0 kommentar(er)
